Silver stain is prone to an effect called “metaling” which occurs as an oxidation on the surface of the glass. It is characterized by a milky opalescence which can have a blue, green or brown cast. It is visible in reflected light. In transmitted light the stain will still appear yellow but with a reduction in transparency. Metaling can be unsightly and can only be removed from the surface of the glass by abrasion or with hydrofluoric acid. The cause of metaling is linked to temperature; the higher the stain is fired the more likely it is to occur. The chemistry of the stain, the chemistry of the glass and the thickness of application also play a role. Some stains are formulated with the addition of copper sulfate to intensify their potency. These are more likely to metal. Of the test samples the amber stains exhibited metaling, although not in the range of their thinner application.
Thursday, September 30, 2010
Wednesday, September 29, 2010
Temperature Test - Color Follows Ion Exchange
The final test of my study demonstrates a chemical reaction described by W. A. Weyl, Coloured Glasses, ISBN 0-900682-06-X. He states that the surface exchange of silver ions with sodium ions in the glass begins at 752°F, but crystals of silver atoms, which transmit yellow light, don't form until higher temperatures are reached. Basically, the silver penetrates the glass even before we see any change in color. In this test 2 samples of glass were fired to 800°F and held at temperature for 20 minutes. The clay body of the stain was washed off and one sample was fired again to 1050°F and held for 2 hours. The upper tiles are the tin side of float and the lower tiles are Lamberts. Comparing these results with the previous test (which was also fired to 1050°F) reveals the role the clay body plays in the reaction. The purpose of the clay or ochre binder, besides suspending the silver oxide, is to capture the resulting sodium salt, forcing more of the sodium ions present in the glass to exchange with silver.
 |
| REUSCHE 292465 (Amber H465) |
 |
| REUSCHE 1382 (Orange #2) |
 |
| REUSCHE 1384 (Yellow #3) |
Labels:
glass chemistry,
silver stain,
temperature,
test result
Temperature Tests - Multiple Firings
The object of this test was to discover what happens when glass which has already been stained is fired a second time. The upper sample is the tin side of float glass; the lower sample is Lamberts. Two pieces of each glass were to 1050°F and held for 2 hours. After cooling the clay body of the stain was removed and the right hand sample was fired a second time and held for 1 hour. The effect is subtle but the stain continues to disperse into the glass.
 |
| REUSCHE 292465 (Amber H465) |
 |
| REUSCHE 1382 (Orange #2) |
 |
| REUSCHE 1384 (Yellow #3) |
Labels:
glass chemistry,
silver stain,
temperature,
test result
Tuesday, September 28, 2010
Temperature Test - Hold Time
Firing slower or holding for a longer time at temperature will also have an effect. These samples were fired to 1000°F. The left sample was held for 20 minutes; the right was held for 6 hours.
 |
| REUSCHE D292465 (Amber H465) on Float (Tin side) |
 |
| REUSCHE D292465 (Amber H465) on LAMBERTS |
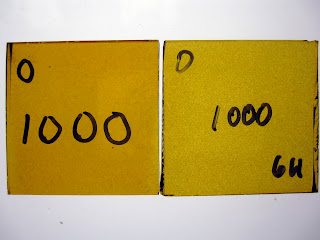 |
| REUSCHE 1383 (Orange #2) on Float (Tin side) |
 |
| REUSCHE 1383 (Orange #2) on LAMBERTS |
 |
| REUSCHE 1384 (Yellow #3) on Float (Tin side) |
 |
| REUSCHE 1384 (Yellow #3) on LAMBERTS |
Labels:
glass chemistry,
silver stain,
temperature,
test result
The Temperature Tests
In the next round of testing I selected 3 stains and made samples fired at 100° F increments from 800° F to 1400°F. The kiln was held at the temperature indicated for 20 minutes. This test reveals how temperature effects the silver stain reaction. The sweet spot appears to take place between 1000° F & 1100°F. In the images below, the upper row of tiles are the tin side of float glass and the lower row are Lambert's glass. The first tile in each group was left unfired. At temperatures above 1300°F the clay body in the stain began to fuse to the glass which accounts for the dark tiles. None of the 1400°F tiles are transparent as the clay body could not be removed. The additional tiles in the 1000°F range are discussed in the next entry.
 |
| REUSCHE D292465 (Amber H465) |
 |
| REUSCHE 1383 (Orange #2) |
 |
| REUSCHE 1384 (Yellow#3) |
Labels:
glass chemistry,
silver stain,
technique,
temperature,
test result
Glass Chemistry & Silver Stain
It was interesting to note that some glasses, like GNA were difficult to stain whereas the tin side of float glass and Bullseye’s “Reactive Ice” were extremely sensitive to the stain. The notes in one text I studied suggested that clear glasses with a blue or green cast would stain better than those with a yellow cast. Another mentioned that clarifying agents added to make glass optically clearer can inhibit the stain. This may explain why historic glasses, which were less “pure”, took the stain better. Of the 3 mouth blown glasses I tested, the glass coming from Poland
 |
| BULLSEYE Clear fusible |
 |
| BULLSEYE "Reactive Ice" |
 |
| Float Glass (non-tin side) |
 |
| Float Glass (tin-side) |
 |
| Desag/Schott GNA |
 |
| KRASNOW |
 |
| LAMBERTS |
 |
| ST. JUST |
 |
| SPECTRUM "System 96" |
 |
| SPECTRUM "Waterglass" |
Labels:
glass chemistry,
manufacturers,
silver stain,
test result
The REUSCHE Stains
 |
| REUSCHE 1382 (Orange #1) |
 |
| REUSCHE 1383 (Orange #2) |
 |
| REUSCHE 1384 (Yellow #3) |
 |
| REUSCHE 1388 (Orange Intense) |
 |
| REUSCHE 1390 (Amber Intense) |
 |
| REUSCHE D292465 (Amber H465) |
Labels:
manufacturers,
REUSCHE,
silver stain,
suppliers,
test result
The DEBITUS Stains
 |
| DEBITUS 20% Silver Chloride |
 |
| DEBITUS 40% Silver Chloride |
 |
| DEBITUS Amber |
 |
| DEBITUS 10% Silver Chloride |
 |
| DEBITUS 20% Silver Chloride |
 |
| DEBITUS 40% Silver Chloride |
Labels:
DEBITUS,
manufacturers,
silver stain,
suppliers,
test result
The Reaction Test - AKA: Silver Stain Smackdown
In the first test, 18 different stains were applied to 10 different clear glasses (from different manufacturers) and fired at the same temperature (1100°F) to reveal the relationship between the chemistry of the stain and the chemistry of the glass. The resulting samples were organized into sets in which these correlations could be clearly seen. One can view these results to learn how a particular stain took on different glasses or how a specific glass reacts to different stains.
Firing Silver Stain
The samples were fired in an electric kiln over a 2 hour period to 1100° F and held at that temperature for 5 minutes.
 |
| Samples are fired "face up" |
 |
| BEFORE: Unfired samples |
 |
| AFTER: Fired samples (before removing clay residue). Note the color change of both the clay and the glass compared to the unfired samples above. |
Labels:
how-to,
kiln,
silver stain,
technique,
test result
Notes about Airbrushing Silver Stain
APPLICATION NOTES:
At the time of application the following observations were made. Ease of straining and applying paint was noted. The amount of gum was judged by stippling with a white hog stippler. If no comment appears about a particular attribute on the chart below it indicates that the performance was average for the group
| Reusche ( | |
| 1382 | Good stipple |
| 1383 | Sprays good, medium gum, stipples good |
| 1384 | Spray OK, stipple OK |
| 1388 | average |
| 1390 | Spray OK, stipple OK |
| D292465 | Sprays good, heavy gum |
| Debitus (FRANCE) | |
| 10% S. Chloride | Gritty, hard to spray |
| 20% S. Chloride | Gritty, hard to spray |
| 40% S. Chloride | Sprays OK |
| 20% S. Sulfide | average |
| 40% S. Sulfide | Very soft, no gum |
| Amber | Very hard, lots of gum |
| Copper Red | Very soft, no gum |
| Oster ( | |
| Ancient | Sprays good, stipples good |
| Ancient | Sprays good, hard to stipple, more gum than others |
| Ancient Lemon | Sprays OK, grittier than others, soft stipple, less gum than others |
| Keracolor ( | |
| 76050 | Mixes well, spray OK, firm stipple- about perfect amount of gum |
| ↑1:4↓ | Mixes well, sprays good, easy to stipple |
| 73028/A | Very soft, little or no gum |
Labels:
airbrush,
manufacturers,
silver stain,
technique,
test result
Applying Silver Stain with an Airbrush
Subscribe to:
Comments (Atom)












